Effective Ways to Calculate Specific Heat in 2025
Understanding and calculating specific heat is vital in various fields such as physics, chemistry, and engineering. Specific heat is defined as the amount of heat needed to change the temperature of a unit mass of a substance by one degree Celsius. In this article, we will explore practical methods for success in calculating specific heat, including useful equations, measurement techniques, and applications in real life.

The Basics of Specific Heat
Before embarking on calculations, it’s essential to grasp the fundamentals of specific heat capacity. The specific heat formula is expressed as:
Q = mcΔT
Where Q is the heat added or removed (in joules), m is the mass (in grams), c is the specific heat capacity (in J/g·°C), and ΔT is the temperature change (in °C). This formula bridges the gap between thermal energy transfer and specific heat, making it foundational for various applications from calorimetry experiments to industrial heating processes.
Understanding Specific Heat Values
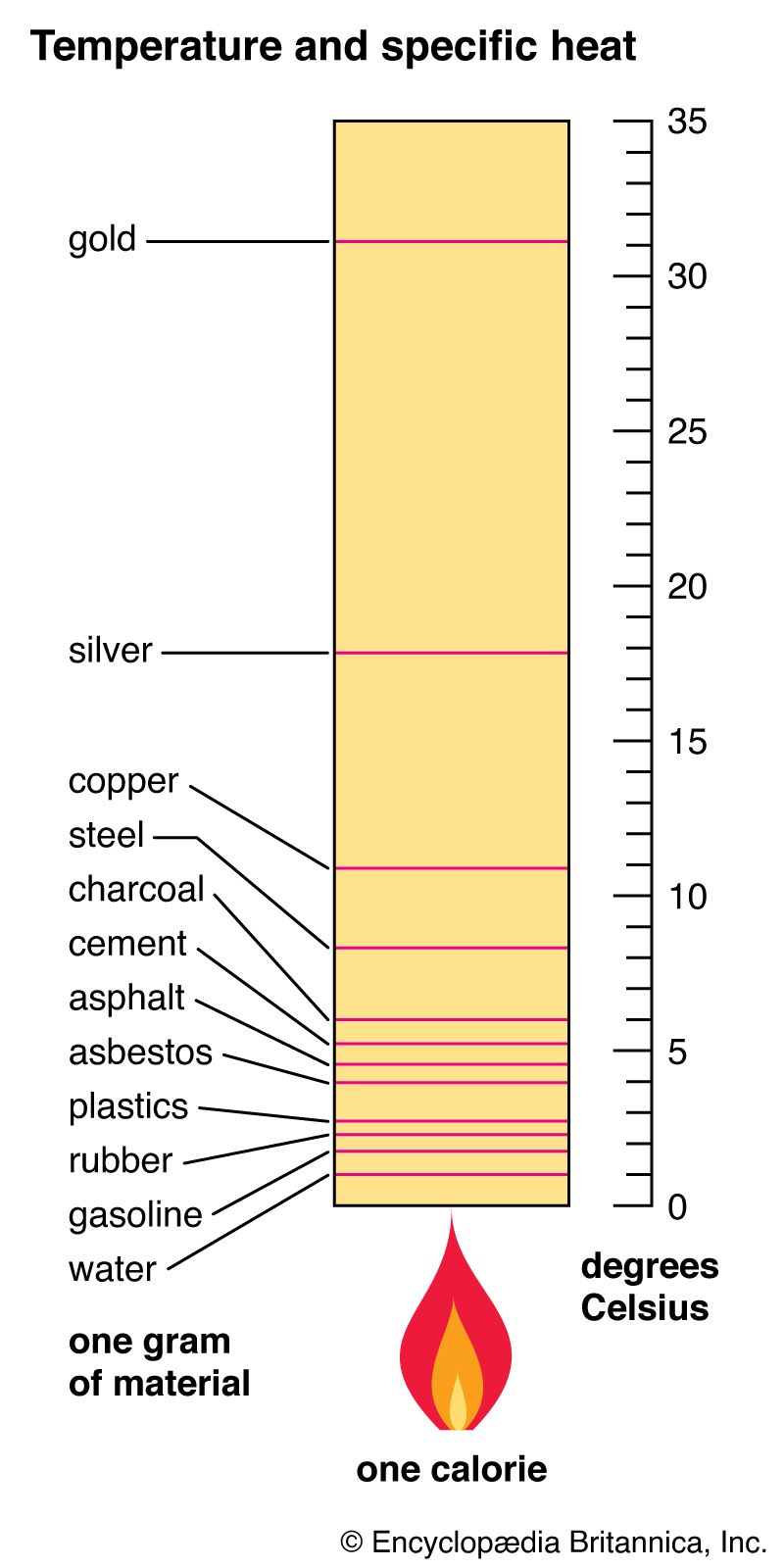
The specific heat of different materials reveals much about their thermal properties. For example, water specific heat is high (approximately 4.18 J/g·°C), making it ideal for temperature regulation in natural and engineered systems. In contrast, metals typically have much lower specific heat capacities; for instance, aluminum is around 0.90 J/g·°C. This difference impacts choices in materials used for applications like cookware, where you require you materials with different thermal management capabilities.
Factors Affecting Specific Heat
Several factors influence a substance’s specific heat capacity. Molecular structure is perhaps the most significant, where simpler molecules tend to have lower specific heat values. Furthermore, transition between solid, liquid, and gaseous states can greatly affect heat transfer. For instance, specific heat in biology often highlights how living entities—primarily composed of water—maintain temperature in varying environments, a critical aspect for phenomena such as homeostasis.
Calculating Specific Heat in Practice
Calculating specific heat in real-world settings can involve various practical methods, depending on the materials and required accuracy. For instance, laboratory measurements can utilize calorimeters, which directly assess heat changes due to temperature variations.
Using Calorimetry for Accurate Measurement
Calorimetry is a common method for determining specific heat capacities with high accuracy. In a basic calorimetry experiment, you’ll heat a substance and monitor the temperature change. The calorimeter provides a controlled environment, allowing for precise calculation of heat adjustments, therefore making it easier to find specific heat values. For instance, if you measure a 50g sample heating from 25°C to 75°C, by incorporating the mass and temperature change into our specific heat equation, you can derive the specific heat capacity of the tested material.
Real-World Example: Calculating the Specific Heat of a Metal
Let’s demonstrate the calculation of metallic specific heat through a step-by-step example. Suppose we have a copper block with a mass of 100g heated sufficiently to increase from 20°C to 80°C, releasing 6000 J of heat. Applying our earlier specific heat formula:
Q = mcΔT
We have:
- Q = 6000 J
- m = 100g
- ΔT = 80°C – 20°C = 60°C
Now rearranging to find c:
c = Q / (m * ΔT) = 6000 J / (100g * 60°C) = 1 J/g·°C. This process outlines a typical laboratory evaluation of metals’ specific heat, showcasing the method’s application in physics.
Applications of Specific Heat Measurement
Specific heat has extensive applications in various fields beyond just laboratory settings. For example, specific heat in cooking illustrates its relevance in culinary practices where adequate heating must be achieved to preserve flavor and texture in food.
Specific Heat in Environmental Science
In environmental science, understanding specific heat aids in analyzing thermal energy absorption rates of various surfaces. Research on urban heat islands utilizes specific heat analysis to determine effective strategies for mitigating heat in cities, showing the implications of specific heat in climate science.
Importance in Engineering Settings
Within engineering, especially in HVAC systems, knowledge of specific heat is indispensable for designing climates that optimize thermal energy transfer. Specific heat measurements inform material selection and system design, ensuring efficiency in both heating and cooling applications.
Key Takeaways
- The specific heat formula expresses the relationship between heat added, mass, and temperature change essential for diverse applications.
- Experimental methods like calorimetry provide precise measurements of specific heat capacity across materials.
- Understanding substances’ specific heat provides insight into material selection in various scientific fields from cooking to engineering and environmental science.
FAQ
1. What is the significance of specific heat in daily life?
The concept of specific heat plays a crucial role in daily life, influencing temperature management in cooking, climate control in buildings, and temperature stability in environments with large bodies of water. By understanding specific heat, we can make informed choices about materials and methods to achieve optimal energy efficiency.
2. How is specific heat related to calorimetry?
Calorimetry is an experimental technique that measures heat transfer changes in a material, allowing for the calculation of specific heat values. This method is critical in both laboratory and industrial applications, where accurate energy measurements are essential for material analysis.
3. Can specific heat be applied in biology?
Yes! The understanding of specific heat in biology helps explain how organisms maintain stable temperatures despite environmental changes. Living beings, primarily composed of water with a high specific heat, can regulate internal temperatures, serving essential functions for metabolic processes.
4. What are the units of specific heat?
Typically, the units of specific heat are expressed in joules per gram per degree Celsius (J/g·°C). In initiated applications, molar heat capacity can also be used, often represented in joules per mole per degree Celsius (J/mol·°C) for substance evaluations.
5. How do specific heat values influence material selection in engineering?
In engineering, knowing a material’s specific heat assists engineers in selecting suitable materials for thermal systems, such as heating and cooling applications. Materials with appropriate specific heat capacities lead to energy-efficient systems, significantly impacting building designs and industrial processes.